Foghorn Therapeutics
Last updated: Mar 4, 2022 9:00 AM EST
CONTENTS
History & Background Management Scientific Advisory Board Board of Directors Ownership Pipeline FHD-286 BAF complexes Opportunity of BAF inhibitors Acute myeloid leukemia FHD-286 pre-clinical data - AML Metastatic uveal melanoma FHD-286 pre-clinical data - UM FHD-286 clinical development FHD-609 Synovial sarcoma/BRD9 dependency FHD-609 pre-clinical data FHD-609 clinical development Early pre-clinical BRM inhibition/degradation BRMi pre-clinical data ARID1B degrader Competition BAF complex inhibitors BRD9 ARID1B Loxo (Eli Lilly) collaboration Merck collaboration Key Intellectual PropertyBackground
Foghorn Therapeutics was founded in 2015 by Flagship Pioneering and world-renowned academics Dr. Cigall Kadoch and Dr. Gerald Crabtree. The company is headquartered in Cambridge, MA.
Dr Kadoch is an institute member of the Broad Institute, an investigator at the Howard Hughes Medical Institute, and an assistant professor of pediatric oncology at the Dana-Farber Cancer Institute of Harvard Medical School. She has a PhD in Cancer Biology from Stanford and has worked extensively on the SWI/SNF (BAF) complexes.
Dr. Gerald Crabtree MD is an investigator at the Howard Hughes Medical Institute and a professor of pathology and developmental biology at Stanford. He is also a co-founder of Ariad Pharmaceuticals and Amplyx Pharmaceuticals. He has worked extensively on characterising BAF complexes since the early 1990s. His lab in 1993 discovered BRG1 as necessary for DNA transcription and mitotic growth1.
In the early 2010s, Dr Kadoch worked in the laboratory of Dr Crabtree to highlight the role and frequency of SWI/SNF (BAF) subunit mutations in cancer. They reported the presence of SWI/SNF subunit mutations in 19.6% of malignancies, supporting the ongoing efforts to target these proteins for potentially selective therapies2. This work, underpinned by proteomics and bioinformatics led to the establishment of a drug discovery platform (called Gene Traffic Control) in collaboration with a Flagships Labs team at Flagship Pioneering. The combined efforts led to the formation of Foghorn Therapeutics, with financing led by Flagship Managing Partner and Foghorn co-founder, Dr Douglas Cole. The company has since been developing drugs that target proteins of the chromatin regulatory system (specifically BAF complex members) for the treatment of genetically defined cancers. They have over 100 employees.
In October 2020, an IPO was completed raising $120 M for an initial market cap of ~$570 M.
Management
Much of the management is linked to big pharma, Agios and Flagship. Below are some of the key individuals.
The President & CEO is Adrian Gottschalk, former SVP Therapeutic Area Head for Neurodegeneration at Biogen. He held various positions at Biogen between 2004-2017 and has extensive commercialization experience. He has been the CEO of Foghorn since 2017.
The CMO is Dr Samuel Agresta, MD, MPH & TM, joining in 2019. He is the former CMO of Infinity Pharmaceuticals (2018-2019). Prior to Infinity, he was VP Head of Clinical Development at Agios, leading development of Tibsovo and Idhifa. Prior to Agios, he held positions at Merrimack and Genentech. He was also Associate Medical Director in the Sarcoma Department at Moffitt Cancer Center from 2004-2019.
The CSO is Steven Bellon, PhD, since January 2022. He was promoted from SVP of Drug Discovery after prior CSO Carl P. Decicco retired from his role. Carl Decicco was SVP, Head of Discovery at Bristol-Myers Squibb (2013-2018) and a venture partner at Flagship Pioneering (2018-present). Steven Bellon joined Foghorn in 2016 as head of drug discovery and was previously the head of structural biology, lead discovery and project management at Constellation Pharmaceuticals. He co-led Constellation's bromodomain platform in collaboration with Genentech and was a member of the portfolio management committee. He has a PhD in physical chemistry from MIT under the direction of world-renowned chemist Stephen Lippard with post-doc training at Yale under Nobel Laureate Thomas A. Steitz.
VP, Protein Degrader Platform, Danette Daniels, PhD, since February 2022. She joined from Promega Corporation where she was R&D group leader of functional proteomics, working extensively in targeted protein degradation and co-developing a new PROTAC modality.
VP, Biology, Ryan Kruger, PhD joined Foghorn in March 2021. Prior to Foghorn, he was the VP, head of cancer epigenetics research at GSK.
VP, Chemistry, David Millan, PhD, joined from Forma Therapeutics where he was the head of medicinal chemistry. A small plug for the Forma report which can be read here.
VP, Regulatory Affairs, Jacqueline Cinicola, joined in July 2020. She was the senior director of regulatory affairs at Agios for 7 years. She has a total of 30 years experience, leading 8 IND applications and the NDA that led to Tibsovo's approval.
Scientific Advisory Board
Dr Charles Sawyers, M.D., pioneering oncologist, chair of Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer center, Howard Hughes Medical Institute investigator and past president of AACR.
Dr David Schenkein, M.D., General Partner and co-leader of Google Ventures life science team and former CEO of Agios. He is a hematologist and medical oncologist and was the SVP of clinical hematology/oncology at Genentech and adjunct clinical professor of medical oncology at Stanford. Prior to Genentech, he was SVP of Clinical Research at Millennium Pharmaceuticals.
Dr Tony Kouzarides, PhD, F.Med.Sci., FRS, professor of cancer biology at the University of Cambridge and deputy director of the Gurdon Institute in the UK. He is a co-founder of Abcam, Chroma Therapeutics and STORM Therapeutics.
Dr Gerald Crabtree, M.D. He is a co-founder of Foghorn, Howard Hughes Medical Institute Investigator and Professor at Stanford University. He has close ties to Cigall Kadoch and has led pioneering work on the BAF complex for decades.
Board of Directors
Dr Douglas Cole MD, Flagship Managing Partner and co-founder of Foghorn. Also a co-founder of numerous biotech companies, such as Syros Pharmaceuticals, Moderna, and led investments in other companies such as Agios, Avedro, Denali, Editas, Receptos among others. He is currently on the Board of Denali, Cygnal, KSQ Therapeutics, Sana Biotechnology, Sigilon, Repertoire Immune Medicines and Inzen Therapeutics. He is a neurologist by training and and was an instructor in neurology at Harvard prior to joining Flagship in 2001.
Dr Adrian Gottschalk, President & CEO of Foghorn.
Dr Cigall Kadoch, PhD. Assistant professor of pediatric oncology at the Dana-Farber Cancer Institute, assistant professor of biological chemistry and molecular biology at Harvard and Institute member and co-director of epigenomics program at Broad Institute of MIT/Harvard. She has a PhD in cancer biology from Stanford and was selected as a Howard Hughes Investigator in September 2021 for a 7-year term.
Dr Scott Biller, PhD, strategic advisor to Agios and prior Agios CSO 2010-2019. Also on the Scientific Advisory Board of Vividion, and on the Board of ROME Therapeutics, and Remix Therapeutics. He has thirty years of drug discovery and development experience with contributions to the development of Juxtapid, Onglyza, Farxiga (metabolic disorders) and Idhifa, Tibsovo (mIDH1/2 AML). He has a PhD in Organic Chemistry from Caltech.
Dr Simba Gill, PhD, President & CEO of Evelo Biosciences and Senior Partner at Flagship Pioneering. He was previously a partner at TPG with leadership roles at Roche and Boehringer Ingelheim. He holds a PhD from King's College, London with work on developing humanized antibodies for cancer.
Dr Adam Koppel, MD, PhD, Managing Director at Bain Capital Life Sciences and former EVP Corp Development and Chief Strategy Officer at Biogen (2014-2016). He graduated magna cum laude from Harvard with an MD/PhD in Neuroscience from UPenn and MBA from Wharton. He is on the board of Solid Biosciences, Dicerna, Trevena & Aptinyx.
Dr Michael Mendelsohn, MD, Chairman & Founder of Cardurion Pharmaceuticals. He is the former SVP and Head of Cardiovascular Research at Merck (2010-2013), and former CSO, founder and executive director of the Molecular Cardiology Research Institute. He has an MD from Harvard with a residency/fellowship completed at Brigham and Women's Hospital.
Dr Ian Smith, Chairman of the Board of Solid Biosciences and Viacyte. He is a former EVP, COO & CFO at Vertex (2001-2019).
Ownership
As summarised below.
| Name | Shares owned | Market Value (m) | % of total |
|---|---|---|---|
| Flagship Pioneering | 12,674,120 | 113 | 30.7% |
| Eli Lilly | 4,000,010 | 36 | 9.7% |
| Cigall Kadoch, co-founder | 3,624,389 | 32 | 8.8% |
| FMR LLC | 2,484,834 | 22 | 6.0% |
| Klarman Family Foundation | 2,139,639 | 19 | 5.2% |
| Artal Group S.A | 1,720,720 | 15 | 4.2% |
| Euclidean Capital | 1,261,261 | 11 | 3.1% |
| Blackrock | 1,147,973 | 10 | 2.8% |
| Adrian Gottschalk | 1,084,981 | 10 | 2.6% |
| Vanguard Group | 844,691 | 8 | 2.0% |
| TOTAL OF LISTED | 30,982,618 | 275 | 75% |
Pipeline
As summarised below.
| Candidate | Mechanism of Action | Indication | Phase |
|---|---|---|---|
| FHD-286 | BRM/BRG1 allosteric inhibitor | r/r AML/MDS, metastatic uveal melanoma | I |
| FHD-609 | BRD targeted protein degrader | Synovial sarcoma, SMARCB1-deleted tumors | I |
| --- | Selective BRM inhibitor | BRG1-mutated cancers | Preclin |
| --- | Selective BRM degrader | BRG1-mutated cancers | Preclin |
| --- | ARID1B targeted protein degrader | SMARCA4-mutant cancers | Preclin |
| --- | Transcription factor disruptor | Cancers | Disc |
FHD-286
BAF complexes & chromatin remodelling
The BAF (BRG-/BRM-associated factor) complex is a heterogenous chromatin remodeling complex made up of multiple subnits. It modulates DNA accessibility to regulate gene expression. It is comprised of either one of two ATPases, SMARCA4 (BRG) or SMARCA2 (BRM), as well as several other core and accessory BAF subunits1.
Its function in regulating gene expression is vast and diverse. Genome-wide mapping of BAF subunits by ChIP-Seq has shown binding of up to 40,000 sites across the genome (Pol II regions, 5' ends of protein-coding genes, CTCF sites or putative enhancers) 3. There are three kinds of BAF complexes - canonical (cBAF), non-canonical (ncBAF) and polybromo-associated (PBAF). The non-canonical BAF complex is a newly characterised complex4 that is especially significant in the case where the cBAF complex has been perturbed (by mutation of one of its components, such as ARID1A/B). In this case, cells become genetically dependent on ncBAF and its components. ncBAF complexes include BRD9, GLTSCR1/1L and SS18/L1. The BRD9 subunit is a necessary component of ncBAF, and an important target of Foghorn.
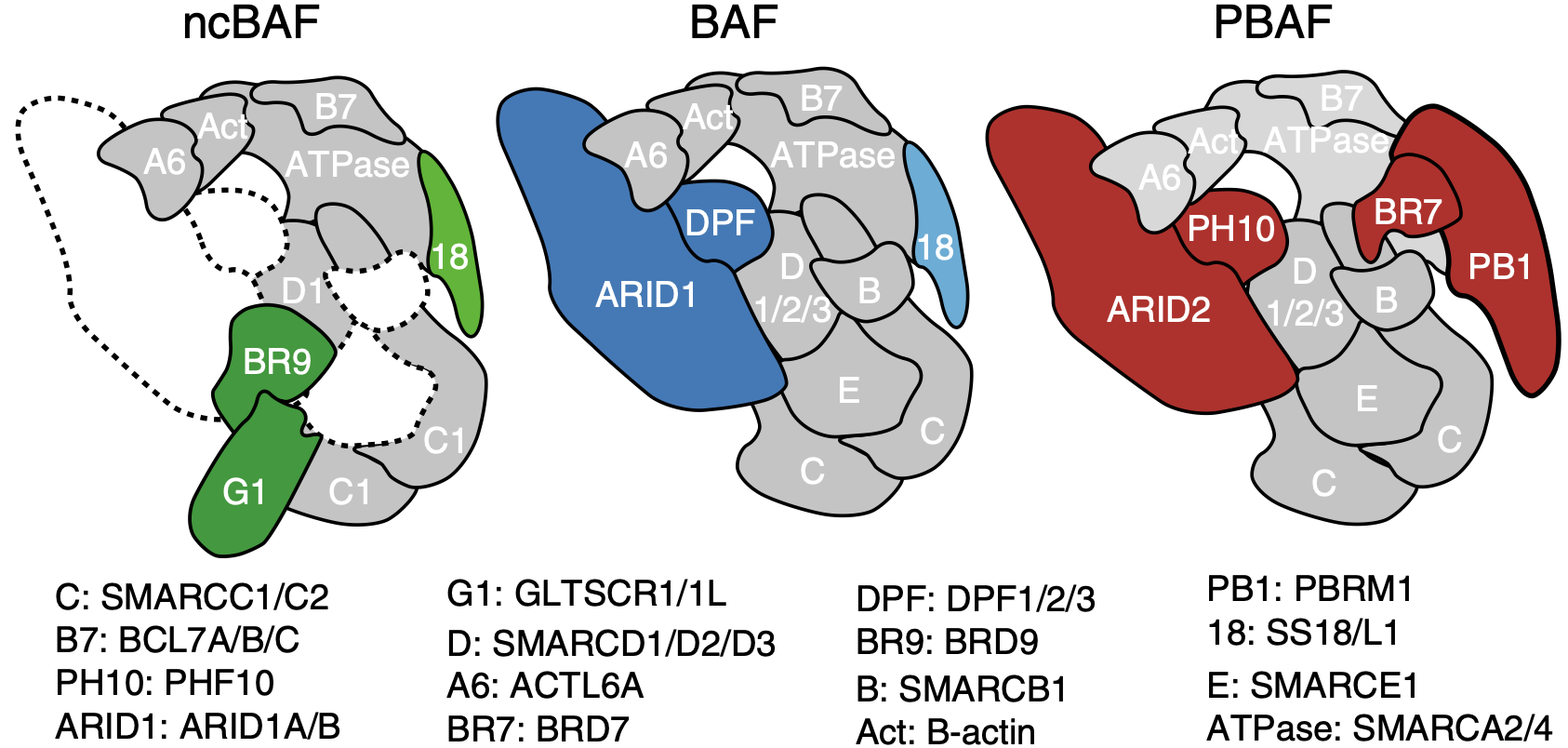
The ATPase is the central piece of the BAF complex. It grasps the nucleosome with its motor engaging directly with nucleosomal DNA to pump DNA for DNA translocation. ARID1A/B stabilizes the base module of the complex serving as a rigid core to the complex. 5.
PBAF complexes are homologous to BAF complexes but are identified with a unique combination of subunits and additional accessory subunits not found in BAF complexes. They contain PBRM1 but lack SS18, and exclusively use BRG (SMARCA4). They also contain BRD7 instead of BRD9, and ARID2 instead of ARID1A/B. Note that ARID2 is not a paralog of ARID1A/B and is distinct in structure. PBAF complexes also contain PHF10 (BAF45A) instead of DPF1/2/3 (BAF45B/C/D).
The genes that encode the subunits of the BAF complex are frequently mutated. Up to 20% of malignancies harbor mutations in BAF/PBAF subunits2. Cells with mutated BAF complexes have disrupted chromatin structures and dysregulated gene expression that promotes survival and cancer progression.
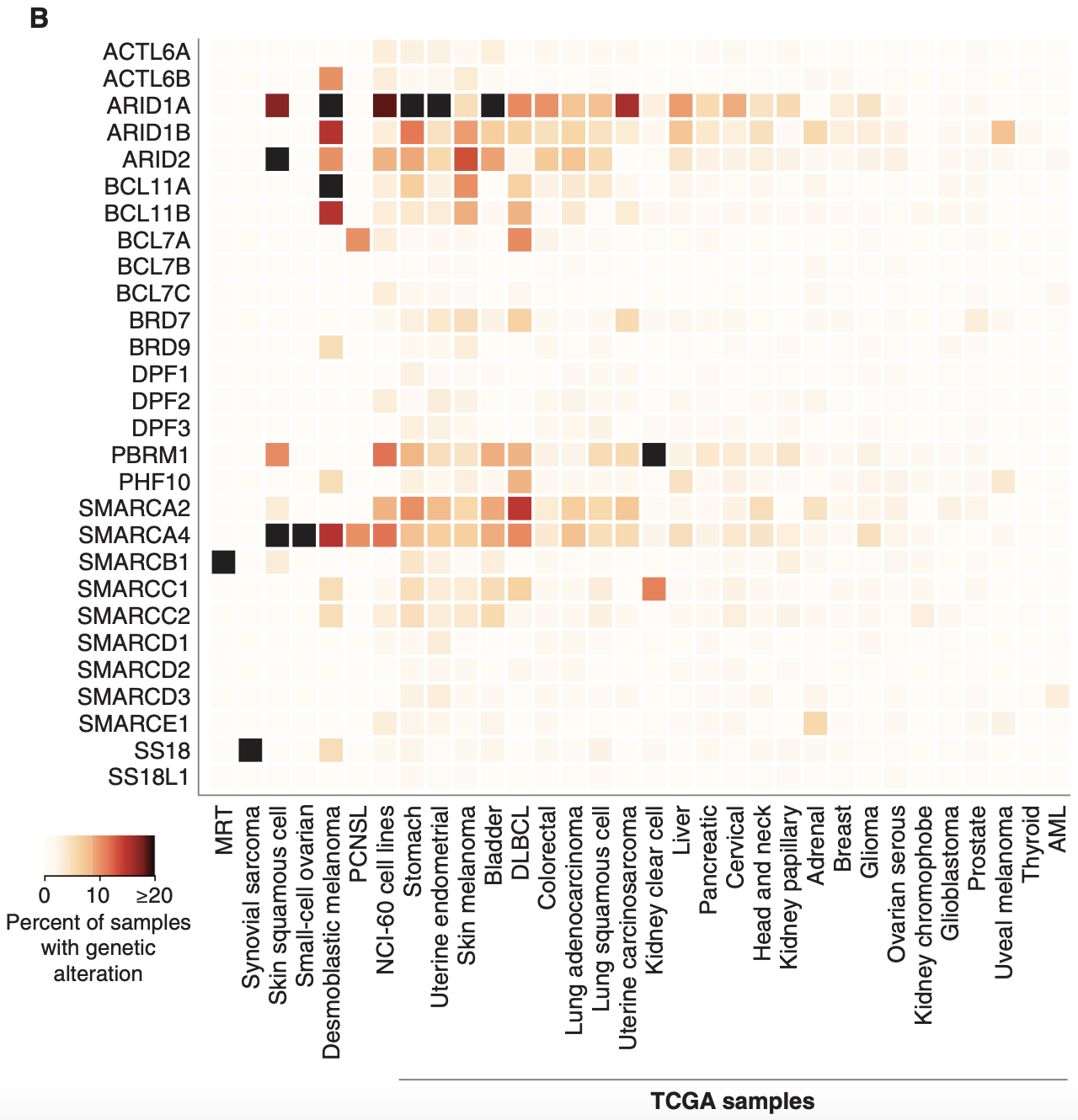
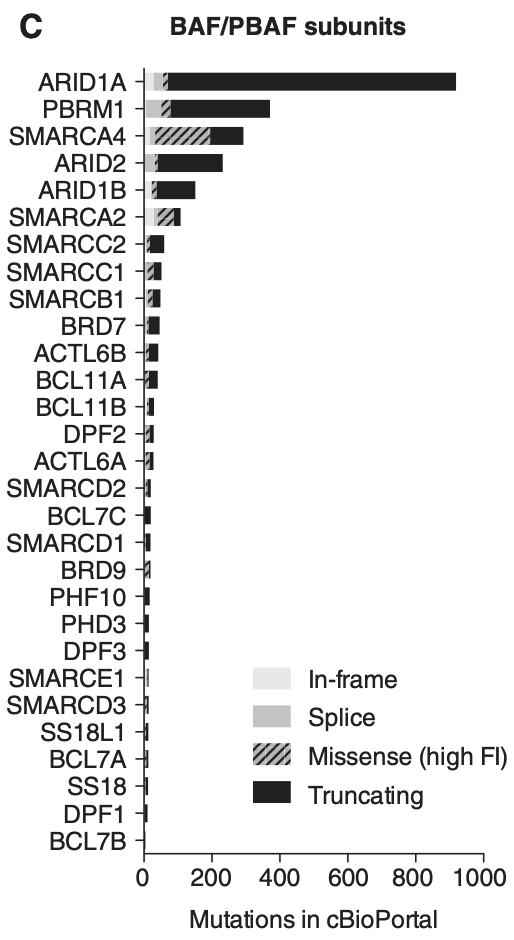
When it comes to the different subunits of BAF complexes, BRG and BRM are frequently mutated with a high number of missense mutations. ARID1A is the most mutated subunit in terms of frequency (30% of malignant ovarian clear cell carcinoma (OCCC), 28% of endometrial endometrioid adenocarcinoma, 21% of endometrial carcinoma, 19% of bladder urothelial carcinoma and urothelial carcinoma, 9% of follicular lymphoma, 8% of GEJ adenocarcinoma, 7.5% of DLBCL, 6% of all malignant solid tumors)7.
However, the effect of ARID1A mutations on BAF function may not be as significant as those that affect BRM/BRG. This is because mutations in ARID1A are most frequently truncating mutations, with the resulting protein likely degraded and thus dissociated from the BAF complex. It is also important to note that mutations typically occur on a single allele and rarely bi-allelic. Note that ARID1B is a paralog and exists in a synthetic lethality relationship with ARID1A, which Foghorn seeks to exploit.
Besides their contribution to cancer, BAF perturbations are also primary drivers of many rare cancers with largely underserved patient populations.
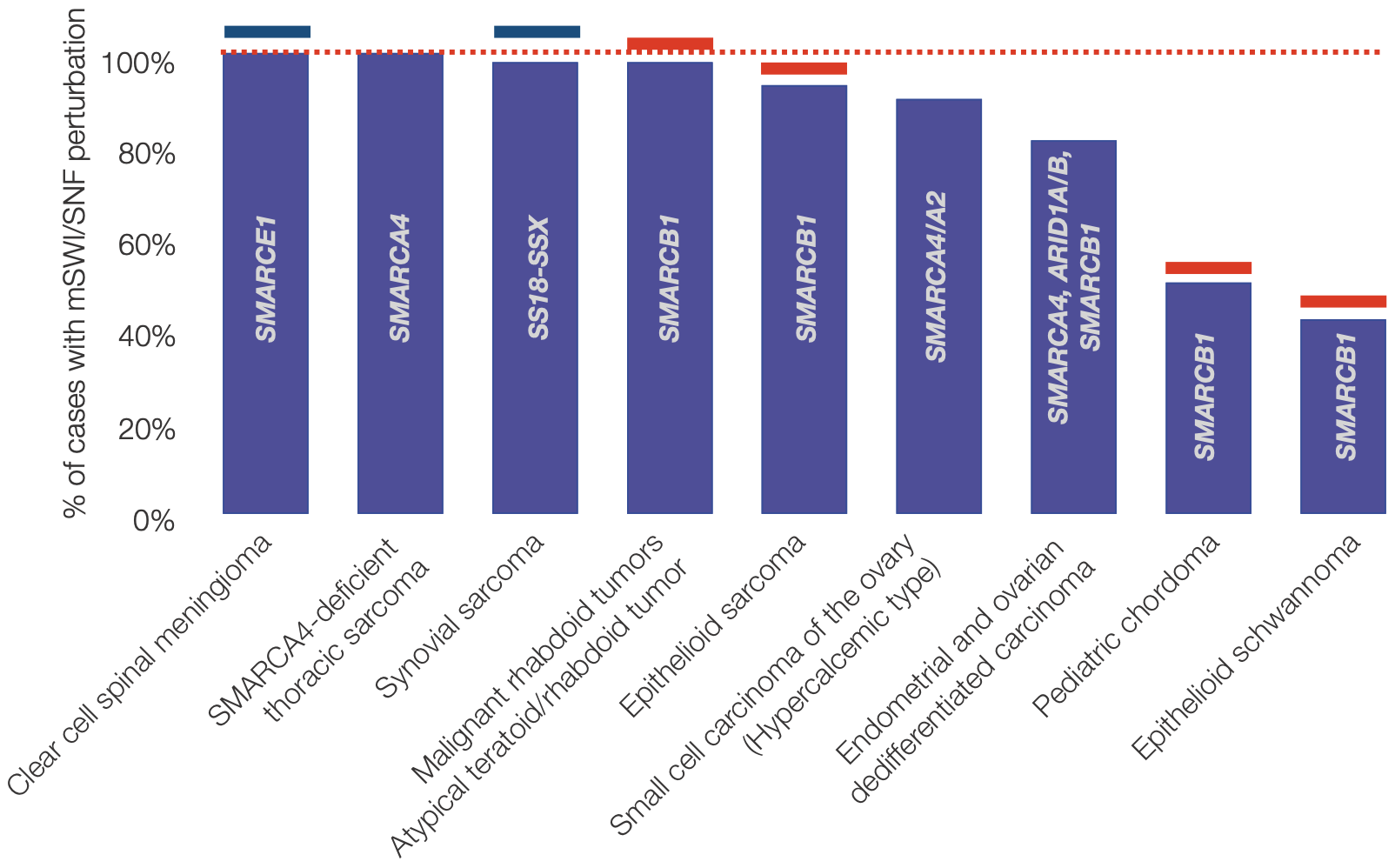
Note that the list of rare cancers above represents approximately ~1000-1200 cases/year in the US given the following: 175 cases/year of clear cell spinal meningioma (given 0.2% of meningioma cases are clear cell spinal 8, 900 cases/year of synovial sarcoma given 7% of soft tissue sarcomas are synovial from 13,000 expected cases in US (cancer.org), 25 case/year of malignant rhabdoid tumor (Boston Children's Hospital and 9), 65 cases/year epithelioid sarcoma given an approximate 0.5% rate of total soft tissue sarcomas 10.
Opportunity of BAF complex inhibitors
As mentioned above, mutations frequently disrupt the subunits of the BAF complex and either contribute to or drive cancer progression. In the context of metastatic prostate cancer for example, 60-70% of cases have an altered BAF profile, typically with elevated BRG1 expression and reduced BRM expression11. In addition and in consequence to the heterogeneity of the BAF complex, various targeting opportunities through synthetic lethality may arise in the context of cancer. For example, BRM/BRG are a synthetic lethality pair. Inhibiting one member of the pair in cells where the other has been made dysfunctional by mutation can have specific and selective effects on these cells while sparing healthy unmutated cells. This could potentially play an important role in achieving therapeutic effects with reduced off-target toxicity.
AML
There are 35,000 cases of acute myeloid leukemia (AML) annually in the major 7 markets (US, EU4, UK & Japan). Recently FDA-approved therapies include treatment with venetoclax, a BH3-mimetic (BCL-2 inhibitor), gemtuzumab ozogamicin, an anti-CD33 ADC, midostaurin (for FLT3+ AML), enasidenib (for mIDH2+ AML) and ivosidenib (for mIDH1+ AML). However, despite the approval of these drugs, five year disease free survival remains at 30-40% with high relapse rates, warranting the development of further treatment options. AML frequently involves the elevated expression of BRG1 in blast cells and BRG1-dependent proliferation. Inhibition of BRG1 can hence reduce BRG1-BAF activity and limit proliferation, inducing terminal differentiation and/or apoptosis. The company hopes to accomplish this with their small molecule BAF inhibitor, FHD-286.
FHD-286 preclinical data in AML
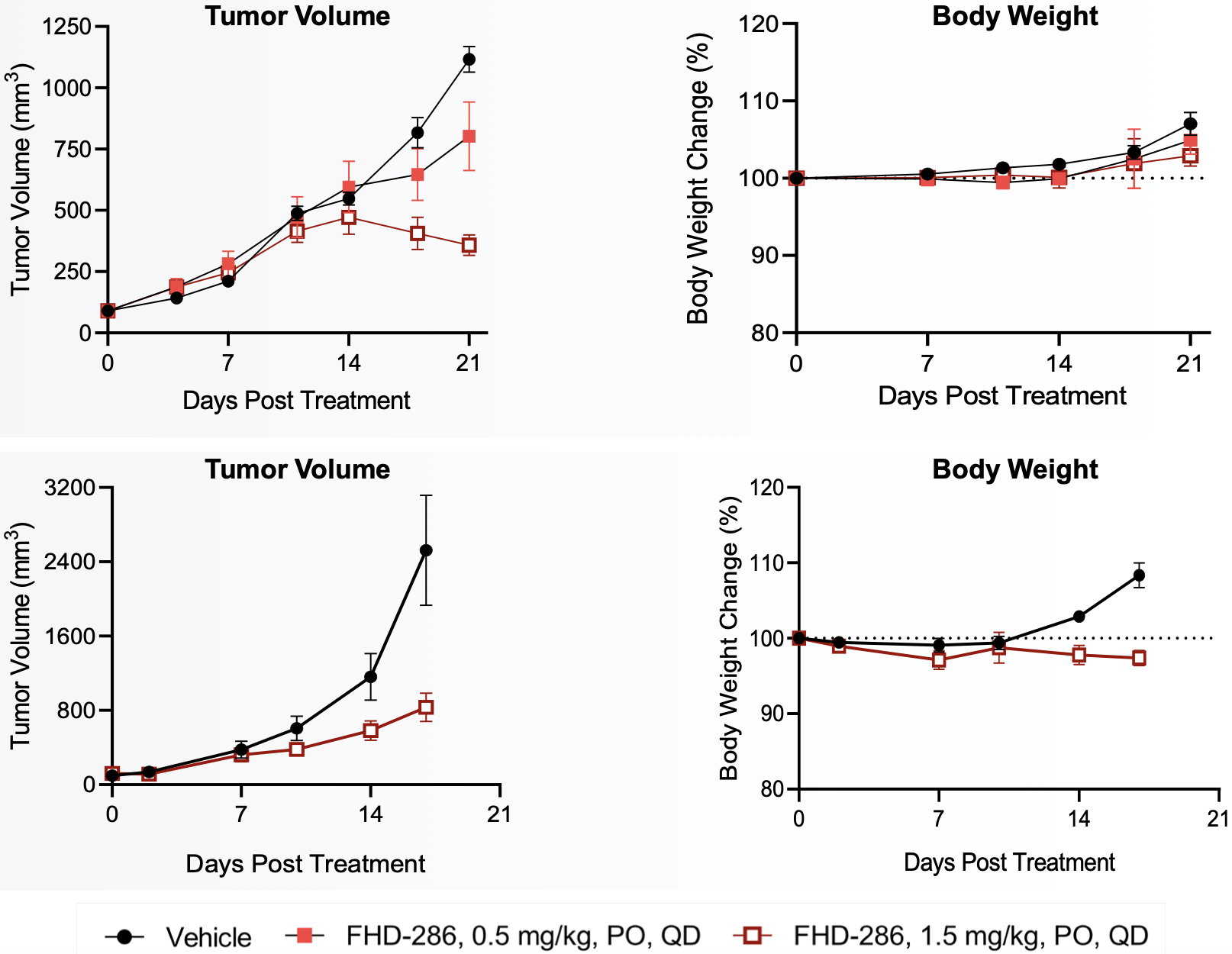
Metastatic uveal melanoma and biological rationale
There are 5,000 cases of uveal melanoma (UM) in the 7 major markets (US, EU4, UK & Japan) with 1700-2500 of these cases occuring in the US12,13. Radiation therapy is typically the first line of therapy and effective in preventing local recurrence in the vast majority of cases. However, metastatic disease is common, occuring in half of all patients and with poor prognoses. There have been no novel treatment options in this space except Tebentafusp (marketed by Immunocore), a bispecific gp100 peptide HLA-directed CD3 T cell engager approved in the US for unresectable or metastatic HLA-A*02:01-positive uveal melanoma on January 25 2022. Tebentafusp received a positive CHMP opinion on February 25 2022.
83-95% of uveal melanomas have GNAQ or GNA11 mutations (FHTX 2021 10-K and 14). The company has established through uveal melanoma cell lines that these mutations confer dependency on the transcription factors MITF and SOX10, which interact abnormally with the BAF complex. Inhibition of BRG1/BRM can hence curtail BAF activity and prevent BAF-associated tumorigenicity. A Novartis Institute group has also shown in pre-clinical models the dependence of uveal melanoma cell lines on members of the BAF complex15. They used two small molecule inhibitors, BRM011 and BRM014 and showed high anti-proliferative activity in numerous uveal melanoma cell lines as well as in an in vivo xenograft model.
FHD-286 pre-clinical data in UM
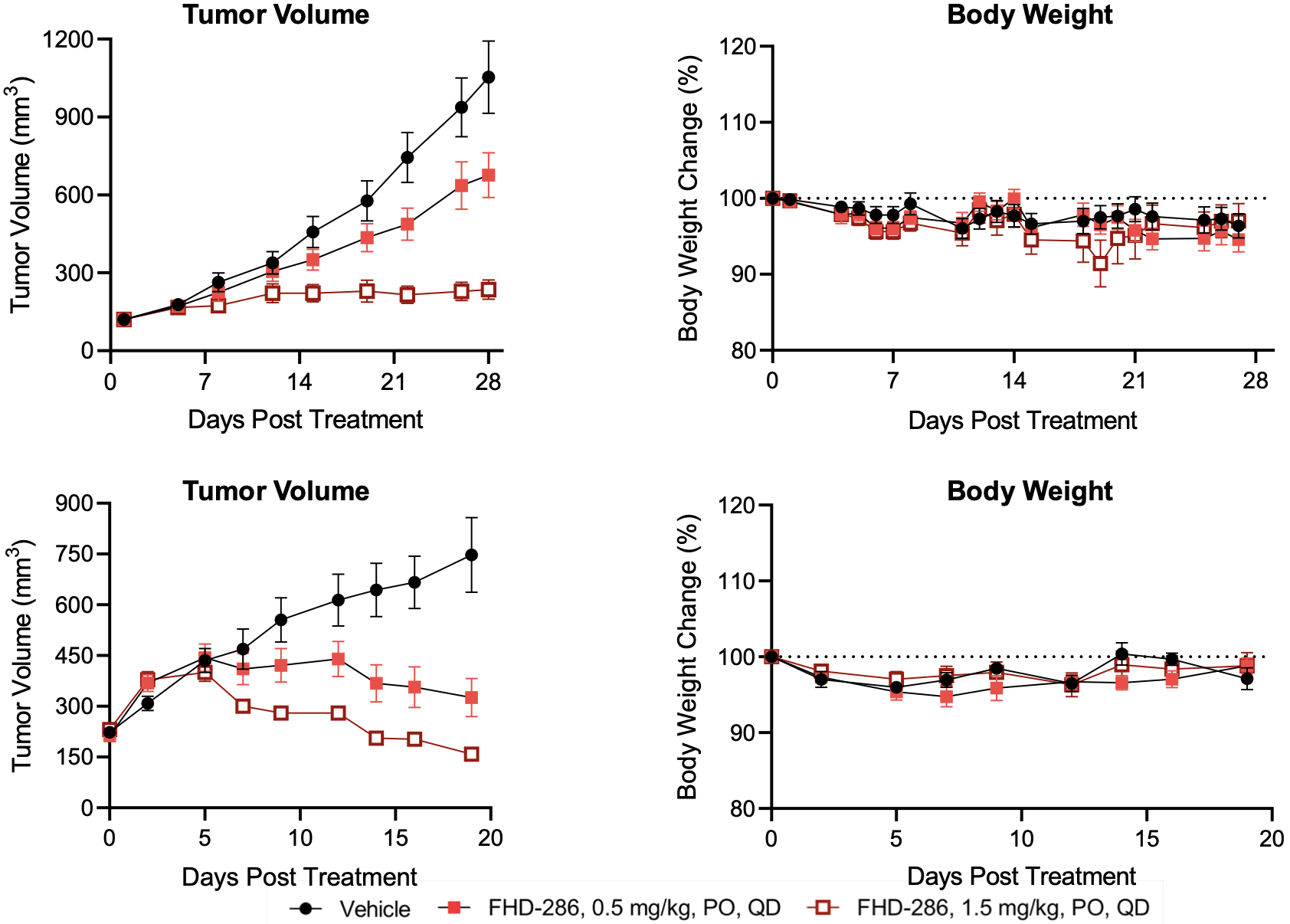
FHD-286 clinical development
Foghorn is currently trialling FHD-286 in two parallel Phase I studies, in patients with r/r AML & MDS (NCT04891757), and in patients with metastatic uveal melanoma (NCT04879017). The trials are designed for single patient accelerated titration to understand PK/PD, before converting to a traditional 3+3 dose escalation design to trial different doses. Following this, expansion cohorts in AML, UM and potentially other indications will be pursued. The first patient was dosed on May 17 2021.
This is a first-in-class drug, so the results will have profound effects on the validation of this mechanism of action. The company anticipates an initial clinical data readout in the first half of 2022.FHD-609
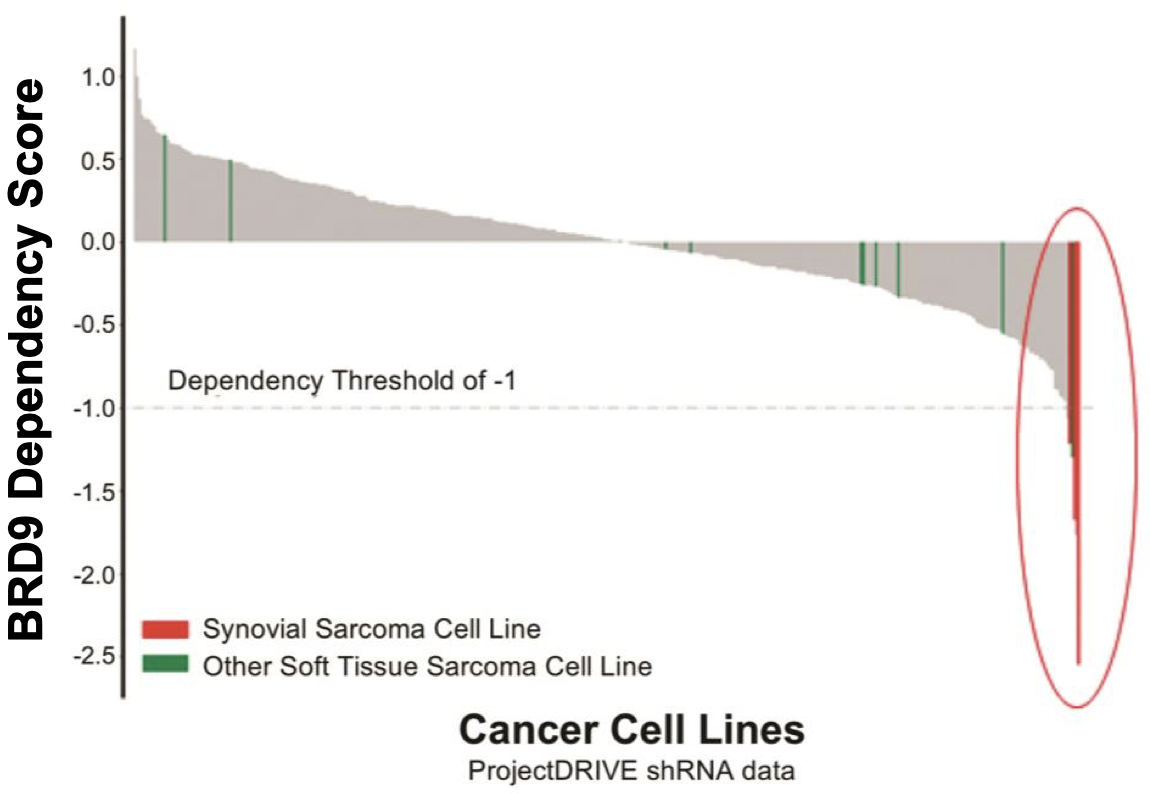
A small molecule BRD9 targeted protein degrader. BRD9 is a component of the ncBAF complex. Certain cancers are dependent on the ncBAF complex (and thus BRD9) for their survival. This can occur in cancers where SMARCB-1 (aka SNF5/BAF47, a component of the cBAF complex) is deleted, or in synovial sarcomas (where SMARCB1 is ejected from the cBAF complex). Cells in these tumors become dependent on the ncBAF complex, of which BRD9 is a necessary component. While small molecule inhibitors to BRD9 exist, their ability to kill synovial sarcoma cancer cells have been questionable or lacking, despite observations of a clear dependency of these cells on BRD9 (when observed with CRISPR screens for example).
Subsequently, Foghorn (and C4 Therapeutics) have seen marked effects preclinically when BRD9 is degraded through targeted protein degradation, especially when compared to BRD9 inhibitors (see C4 Therapeutics 10-K and data below). This would indicate that the inhibition of BRD9 is insufficient in generating a functional or apoptosis-inducing effect, with an advantage to degradation over covalent or non-covalent inhibition.
Foghorn is initially trialling the drug in patients with synovial sarcoma, before potentially expanding into SMARCB-1 deleted tumors. The first patient in the Phase I trial (NCT04879017) was dosed on August 23 2021.
Synovial Sarcoma & BRD9 dependency
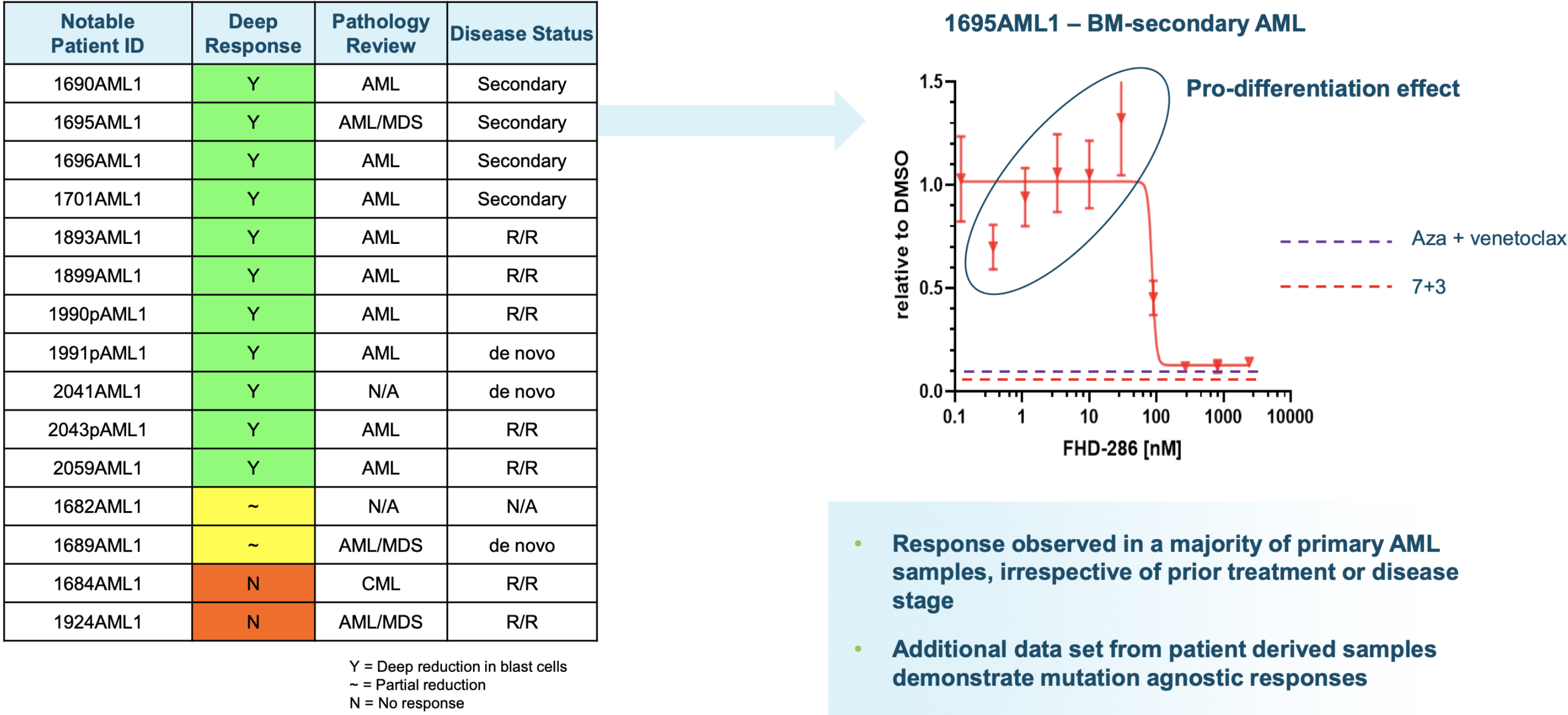
Up to 97% of synovial sarcomas16 are marked by translocations involving SS18. This results in the expression of SS18-SSX oncoproteins (SS18-SSX1 or SS18-SSX2, or more infrequently, SS18-SSX4) and the formation of mutant BAF complexes in which SMARCB1 is ejected (mimicing SMARCB-1 deleted tumors)17. BAF complexes that carry SS18-SSX fusion proteins redirect the complex to oncogenic loci for the expression of a unique SS gene expression signature18, such as the SOX2 and PAX6 genes (pluripotency related transcription factors) that promote tumorigenesis and give the cancer its aggressive stem cell-like presentation6,17. Preclinical data shows synovial sarcoma cell lines have a significantly higher dependency on BRD9 for survival as compared to other soft tissue sarcoma cell types (FHTX data). By degrading BRD9, Foghorn hopes to shut down the effects of SS18-SSX-BAF activity and inhibit tumor growth, promoting better outcomes for these patients.
Another tumor type, rhabdoid tumors (RTs) are genetically characterised by bi-allelic loss of either SMARCB1 (98% of RTs) or SMARCA4 (2%). This similarly results in a reliance on the ncBAF complex and presents as an opportunity for targeting by synthetic lethality. However, treatment of multiple RT cell lines with small molecule BRD9 inhibitors, BI-9564 and I-BRD9 yielded generally none, or at best, mild negative effects on cell proliferation, inducing G1 cell cycle arrest and apoptosis19. Note that these are in vitro results and do not equate to the potential effects of BRD9 degradation.
This hypothesis is supported by preclinical data the company has generated in in vivo models of synovial sarcoma as shown below.
FHD-609 pre-clinical data
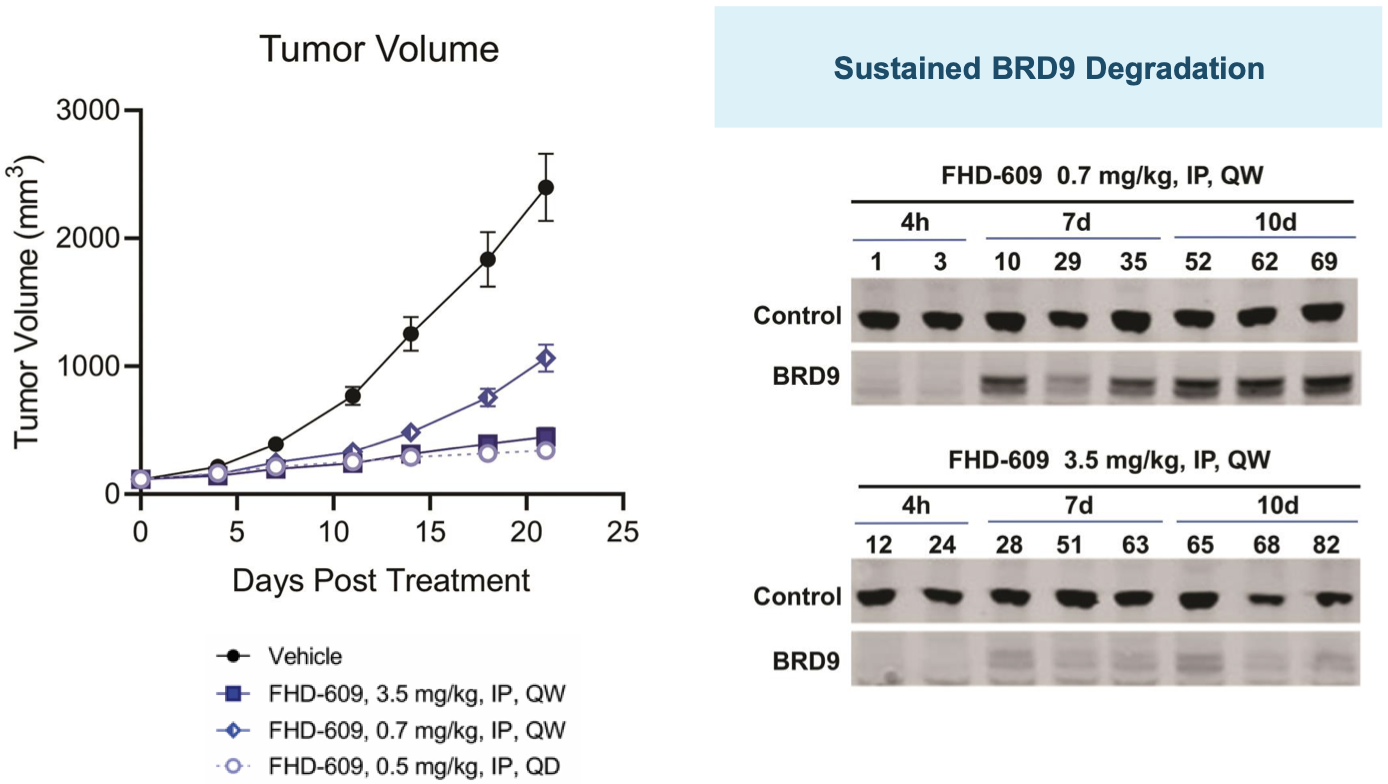
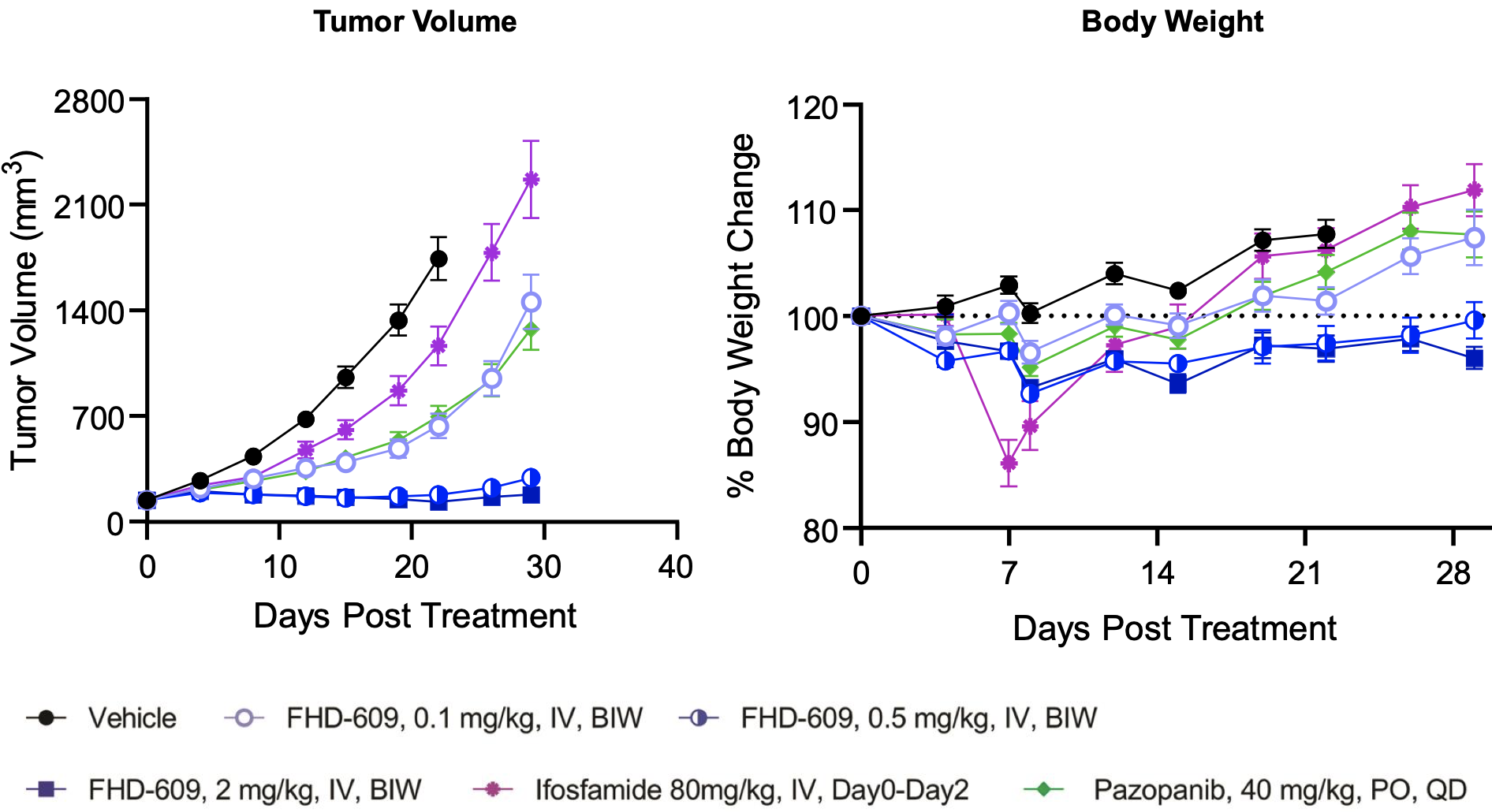
FHD-609 clinical development
An IND was cleared on May 11 2021, and the drug is currently being tested in a Phase 1 trial (NCT04965753) in patients with metastatic synovial sarcoma. The drug is being dosed as a single IV dose given bi-weekly. The first patient was dosed in August 2021, with initial data expected in the first half of 2022.
Early pre-clinical
Selective BRM inhibitor and BRM degrader for BRG1(SMARCA4)-mutated cancers
The company is also developing a BRM inhibitor and a BRM degrader to target cancers with BRG1/SMARCA4 mutations with a synthetic lethality approach. BRG1 is mutated in ~4.6% of all cancers.
| Cancer type | % with mutated BRG1 |
|---|---|
| Endometrial endometrioid adenocarcinoma | 9.9% |
| Medulloblastoma | 8.6% |
| Esophageal adenocarcinoma | 8.6% |
| NSCLC | 8.4% |
| Bladder carcinoma | 7.8% |
| Melanoma | 7.7% |
| GEJ adenocarcinoma | 6.9% |
| Gastric adenocarcinoma | 5.9% |
| Colorectal cancer | 5.6% |
| Malignant solid tumors | 5.0% |
| Non-hodgkin lymphoma | 4.3% |
| Ovarian endometrioid adenocarcinoma | 4.2% |
| HNSCC | 3.9% |
| Ovarian cancer | 3.9% |
| Glioblastoma | 3.0% |
| Breast carcinoma | 2.4% |
| Pancreatic | 2.9% |
The synthetic lethal relationship of BRM-BRG1 and its potential as a target in cancer can be read about extensively in this20 and this21 paper.
In the first study, 16% of 103 surgical specimens of NSCLC tissue revealed complete loss or reduction (by epigenetic silencing) of BRG1. Important to note here that a high proportion of this number is likely epigenetic-related and not a result of mutation. It nonetheless represents a high number of NSCLC patients with BAF perturbations. Interestingly, all 16 of these patients had wild type EGFR, KRAS, DDR2 and were negative for ALK gene fusion and FGFR1 gene amplification, indicating that these patients would not be eligible for many of the precision oncology drugs currently approved and in development for NSCLC, such as the increasingly popular mutant-EGFR inhibitors and KRAS inhibitors (link here for a list of mutant KRAS inhibitors approved and in development). However, an important point is that some of these patients also presented with concomitant loss or reduction of BRM expression (5.8% of total patients were BRG1 AND BRM negative), with BRM deficiency also being likely epigenetically-related. It is possible that these cells have acquired resistance to both BRG1 and BRM depletion through other means and are able to survive without either, posing a problem for treatment by BRM depletion. Nonetheless, 10% of cases expressed BRM but were BRG1 deficient, indicating a high number of patients that could potentially respond to BRM-depleting therapy.
The second paper from the Novartis Institutes also presents evidence of anti-proliferative activity of BRM depletion in BRG1-mutant cancer cells, with translational data in in vivo NSCLC xenograft models. More recently, a paper was published by the same group highlighting the discovery of small molecule BRM inhibitors, with similar anti-proliferative activity in BRG-1 mutant NSCLC xenograft models22.
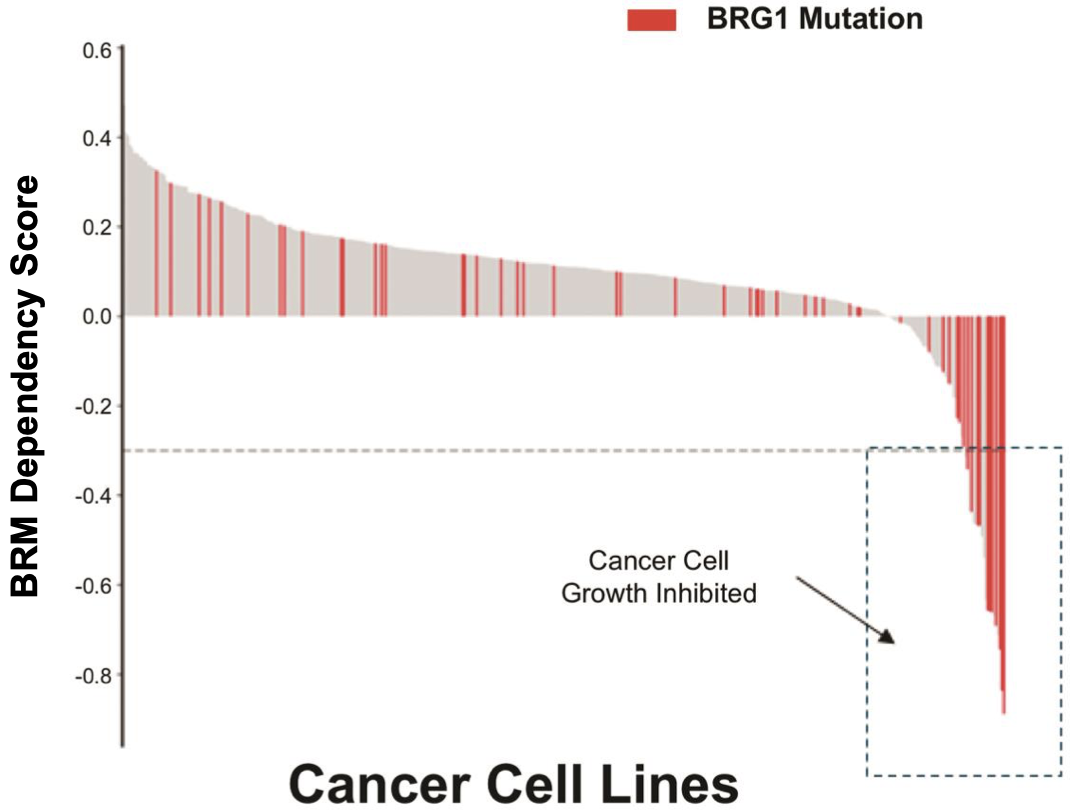
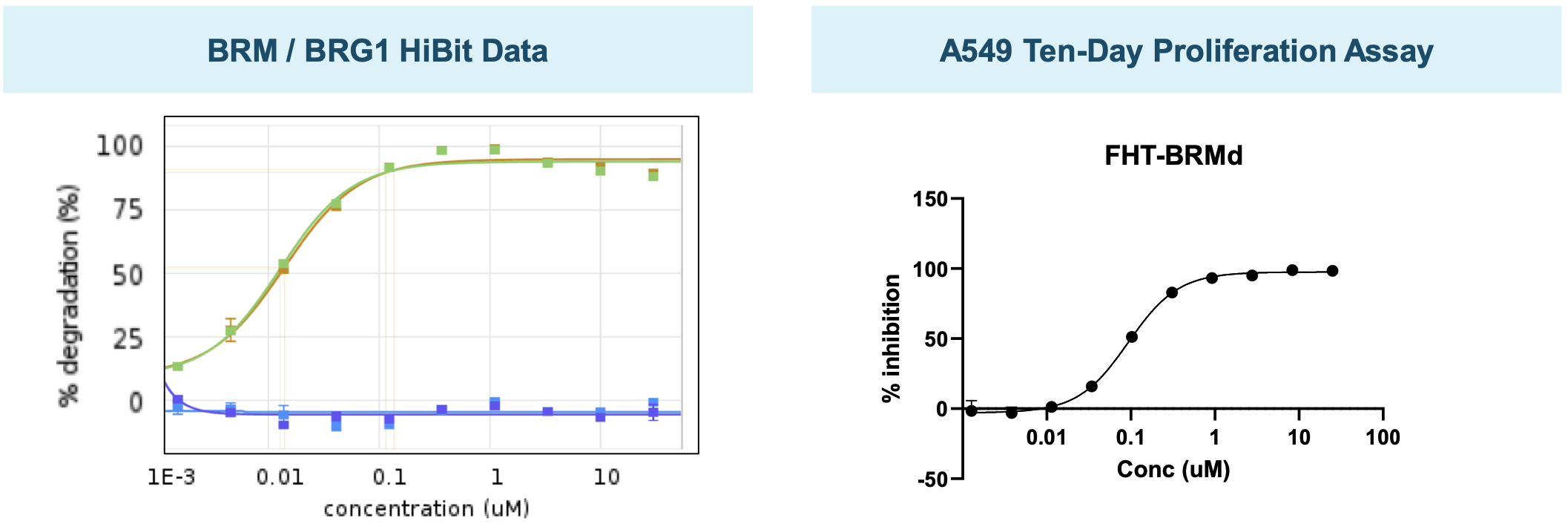
BRMi and BRM degrader pre-clinical data
The company has presented in vivo efficacy data for their BRM-selective inhibitor and BRM-selective degrader.
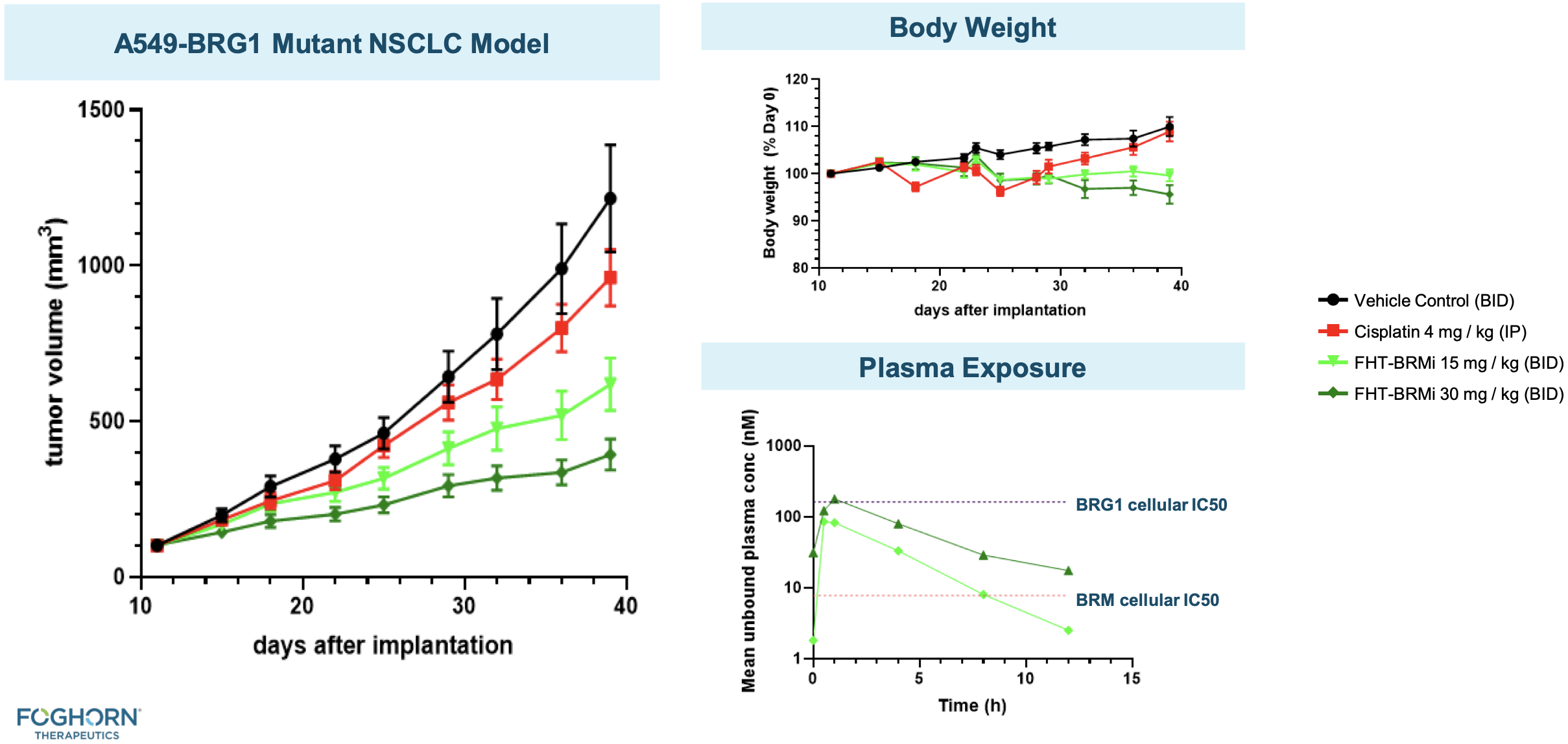
ARID1B degrader
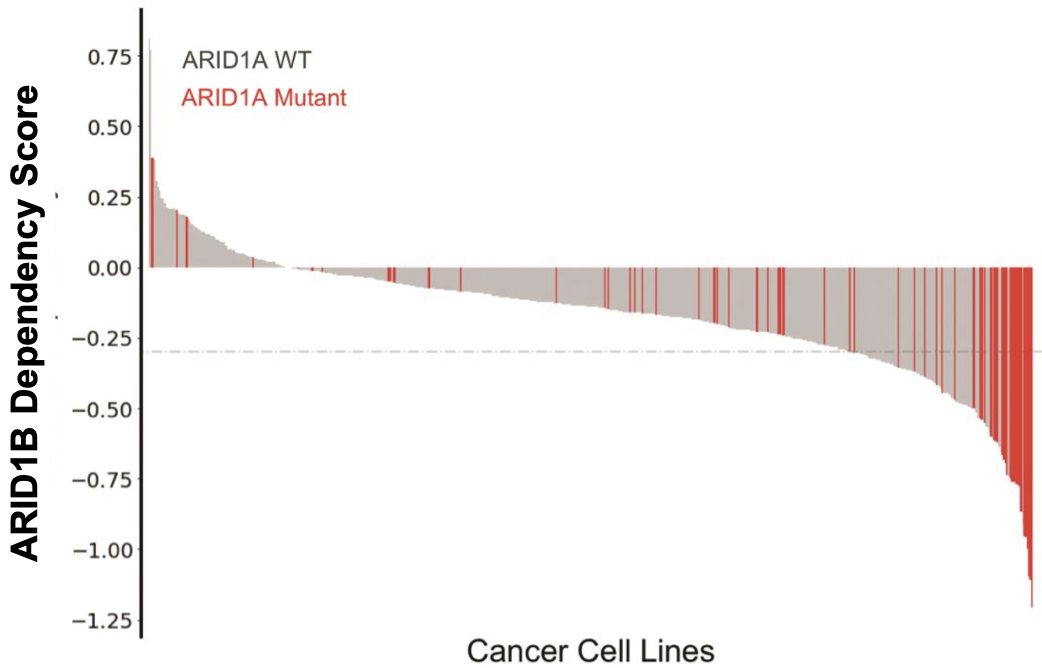
The company also has an ARID1B degrader in early development for the treatment of ARID1A cancers. ARID1A is the most frequently mutated subunit in the BAF complex. In cells with mutated ARID1A, ARID1B plays a compensatory role to maintain BAF complex activity. Note that as of early 2022, this program is now part of the Lilly collaboration.
Competition
BAF complex inhibitors and related competition
There are no other direct BRM/BRG inhibitors in clinical trials besides FHD-286 as far as my knowledge goes. Kadoch's work has been pioneering, and this is the first clinical attempt to target BRM/BRG. Preclinically, Prelude Therapeutics has a BRM (SMARCA2) degrader in development with an IND being filed in 2022. For more about Prelude, click here or search through the "Reports" tab. Ryvu Therapeutics was also developing a SMARCA2 degrader, but they have removed it from their pipeline.
Other BRM/BRG inhibitors exist in the literature, such as the aforementioned small molecule drugs being developed at the Novartis Institutes, BRM011 and BRM014.
Another is ACBI1, a PROTAC degrader of SMARCA2, SMARCA4 and PBRM1. Boehringer Ingelheim has contributed to the pre-clinical work relating to this compound. A paper published in 2019 regarding ACBI1 can be accessed here. There have been no data published from in vivo studies.
BRD-K98645985 is an inhitor of ARID1A-specific BAF complexes. The related paper can be read here.
BRD9-related competition
Like Foghorn, C4 Therapeutics is also developing a BRD9 PROTAC degrader, CFT 8634, for the treatment of synovial sarcoma and SMARCB1-deleted tumors. An IND has been cleared, and the Phase I trial is expected to initiate in the first half of 2022.
GSK has a BAZ2/BRD9 inhibitor that they have not pursued clinically, GSK2801, with a paper that can be accessed here.
Boehringer Ingelheim has two small molecule BRD9/BRD7 probes, BI-9564 and BI-7273 that have been used in research.25
ARID1B competition
ARID1A also exists in a synthetic lethal relationship with various other proteins, such as EZH2. Hence there are various competing molecules targeting ARID1A-mutated tumors. For example, Tazemetostat is an FDA approved EZH2 inhibitor currently in Phase 2 for solid tumors with ARID1A mutations (NCT05023655). The trial began recruiting patients in January 2022.
GSK also has an EZH2 inhibitor, GSK126, which has shown anti-proliferative effects on ARID1A-mutated ovarian clear cell adenocarcinoma cells in vitro24.
ARID1A loss has also been shown to increase sensitivity to other inhibitors, such as those that target ATR, GSH, HDAC2/6, PARP1, PI3K/AKT and YES1.
Loxo (Eli Lilly) collaboration
In December 2021 Foghorn entered into a collaboration deal with Loxo Oncology (Lilly) for the co-development and co-commercialisation of Foghorn's selective BRM program and an additional undisclosed program. The terms of the deal include a 50/50 share in the US economics for the selective BRM program, with Foghorn eligible to receive royalties on ex-US sales in the low double digit range and escalating into the twenties based on revenue levels. For the additional discovery programs, Foghorn may receive up to $1.3 B in potential development and commercialization milestone payments with an option to participate in a percentage of the US economics and to receive ex-US tiered royalties in the mid-single digit to low-double digit range. The option may be exercised after the successful completion of toxicology studies. There are currently three undisclosed discovery programs. Foghorn received an upfront payment of $300 M in cash and an equity investment of $80 M in Foghorn common shares at a price of $20 per share.
Merck collaboration
In July 2020, Foghorn entered into a research collaboration deal with Merck for the identification, validation and delivery of transcription factor disruptors. Under the terms of the agreement, Merck has an exclusive worldwide license to any drugs that arise out of the collaboration. Foghorn received an upfront payment of $15 M and is eligible to receive up to $410 M in milestone payments by any product generated from the collaboration ($245 M in R&D and regulatory milestones and $165 M in sales milestones). Foghorn is also eligible for tiered royalties on net sales in the mid-single digit to low teen range, in the case of product approval and commercialisation.
Key Intellectual Property
As summarised below.
| Asset | Patent type & number | Earliest expiry | Jurisdiction |
|---|---|---|---|
| FHD-286 | COM/MOU (US 20210038611 & US 20210230154 & US 20220016083) | 2039-2041 | US & PCT pending |
| FHD-609 | COM/MOU (US 20210230190 & US 20210009568 /A1 & US 20220048906 & US 20210251988) | 2039-2041 | US & PCT pending |
| ARID1A degrader | COM/MOU (US 20210171958) | 2039 | US & PCT pending |
| SMARCD1 inhibitor & degrader | COM/MOU (US 20210260171) | 2039 | US pending |
| BICRA (GLTSCR1) inhibitor & degrader | COM/MOU ((US 20210251988) | 2039 | US pending |
Disclosure: I am a shareholder of Foghorn Therapeutics as of the time of the publishing of this article. I may exit from this position at any given time without notice. Data presented in this article have been obtained from third-party publications and sources. I do not guarantee the accuracy of this data and all information presented here should be checked and verified accordingly. Readers of this article should each make their own evaluation and judgement of the mentioned companies and of the relevance and adequacy of the information provided. Readers should make other such investigation as deemed necessary. The article is intended for informational purposes. Investors and potential investors are requested to do additional research before investing in any of the companies mentioned in this article. All investments have risks of loss associated with them. Investing is very risky, highly speculative, and should not be done by anyone who cannot afford to lose the entire value of investment and without prior due diligence. Investors should evaluate the risks associated with each individual company before investing. All forward-looking statements are anticipations subject to risks and uncertainties and actual results can differ materially from those projected.
Additional disclosure: This article contains trademarks, trade names, copyrights and data of Foghorn Therapeutics and other companies, which are the property of their respective owners.
Tweet
References
1. Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993 Nov 11;366(6451):170-4. doi: 10.1038/366170a0. PMID: 8232556.
2. Kadoch, C., Hargreaves, D. C., Hodges, C., Elias, L., Ho, L., Ranish, J., & Crabtree, G. R. (2013). Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nature genetics, 45(6), 592–601. https://doi.org/10.1038/ng.2628
3. Euskirchen, G. M., Auerbach, R. K., Davidov, E., Gianoulis, T. A., Zhong, G., Rozowsky, J., … Snyder, M. (2011). Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches. PLoS Genetics, 7(3), e1002008. doi:10.1371/journal.pgen.100200
4. Michel, B. C., D'Avino, A. R., Cassel, S. H., Mashtalir, N., McKenzie, Z. M., McBride, M. J., Valencia, A. M., Zhou, Q., Bocker, M., Soares, L., Pan, J., Remillard, D. I., Lareau, C. A., Zullow, H. J., Fortoul, N., Gray, N. S., Bradner, J. E., Chan, H. M., & Kadoch, C. (2018). A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nature cell biology, 20(12), 1410–1420. https://doi.org/10.1038/s41556-018-0221-1
5. He, S., Wu, Z., Tian, Y., Yu, Z., Yu, J., Wang, X., … Xu, Y. (2020). Structure of nucleosome-bound human BAF complex. Science, 367(6480), 875–881. doi:10.1126/science.aaz9761
6. Hodges, C., Kirkland, J. G., & Crabtree, G. R. (2016). The Many Roles of BAF (mSWI/SNF) and PBAF Complexes in Cancer. Cold Spring Harbor Perspectives in Medicine, 6(8), a026930. doi:10.1101/cshperspect.a026930
7. https://www.mycancergenome.org/content/gene/arid1a/
8. Wang, Y., Qin, X., Liu, M., Liu, X., Yu, Y., Zhao, G., & Xu, Y. (2021). Clear Cell Meningioma in the Central Nervous System: Analysis of Surveillance, Epidemiology, and End Results Database. Frontiers in oncology, 10, 592800. https://doi.org/10.3389/fonc.2020.592800
9. Ostrom, Q. T., Chen, Y., M de Blank, P., Ondracek, A., Farah, P., Gittleman, H., Wolinsky, Y., Kruchko, C., Cohen, M. L., Brat, D. J., & Barnholtz-Sloan, J. S. (2014). The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro-oncology, 16(10), 1392–1399. https://doi.org/10.1093/neuonc/nou090
10. Armah, H. B., & Parwani, A. V. (2009). Epithelioid sarcoma. Archives of pathology & laboratory medicine, 133(5), 814–819. https://doi.org/10.5858/133.5.814
11. Hartley, A., Leung, H. Y., & Ahmad, I. (2020). Targeting the BAF complex in advanced prostate cancer. Expert Opinion on Drug Discovery, 16(2), 173–181.doi:10.1080/17460441.2020.18216
12. Aronow, M. E., Topham, A. K., & Singh, A. D. (2017). Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013). Ocular Oncology and Pathology, 4(3), 145–151. doi:10.1159/000480640
13. curemelanoma.org
14. Shoushtari, A. N., & Carvajal, R. D. (2014). GNAQ and GNA11 mutations in uveal melanoma. Melanoma research, 24(6), 525–534. https://doi.org/10.1097/CMR.0000000000000121
15. Rago, F., Elliott, G., Li, A., Sprouffske, K., Kerr, G., Desplat, A., Abramowski, D., Chen, J. T., Farsidjani, A., Xiang, K. X., Bushold, G., Feng, Y., Shirley, M. D., Bric, A., Vattay, A., Möbitz, H., Nakajima, K., Adair, C. D., Mathieu, S., Ntaganda, R., … Jagani, Z. (2020). The Discovery of SWI/SNF Chromatin Remodeling Activity as a Novel and Targetable Dependency in Uveal Melanoma. Molecular cancer therapeutics, 19(10), 2186–2195. https://doi.org/10.1158/1535-7163.MCT-19-1013
16. Dos Santos, N. R., de Bruijn, D. R. H., & van Kessel, A. G. (2000). Molecular mechanisms underlying human synovial sarcoma development. Genes, Chromosomes and Cancer, 30(1), 1–14. doi:10.1002/1098-2264(2000)9999:9999<::aid-gcc1056>3.0.co;2-g
17. Kadoch, C., & Crabtree, G. R. (2013). Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell, 153(1), 71–85. https://doi.org/10.1016/j.cell.2013.02.036
18. McBride, M. J., Pulice, J. L., Beird, H. C., Ingram, D. R., D'Avino, A. R., Shern, J. F., Charville, G. W., Hornick, J. L., Nakayama, R. T., Garcia-Rivera, E. M., Araujo, D. M., Wang, W. L., Tsai, J. W., Yeagley, M., Wagner, A. J., Futreal, P. A., Khan, J., Lazar, A. J., & Kadoch, C. (2018). The SS18-SSX Fusion Oncoprotein Hijacks BAF Complex Targeting and Function to Drive Synovial Sarcoma. Cancer cell, 33(6), 1128–1141.e7. https://doi.org/10.1016/j.ccell.2018.05.002
19. Krämer, K., Moreno, N., Frühwald, M., & Kerl, K. (2017). BRD9 Inhibition, Alone or in Combination with Cytostatic Compounds as a Therapeutic Approach in Rhabdoid Tumors. International Journal of Molecular Sciences, 18(7), 1537.doi:10.3390/ijms18071537
20. Oike, T., Ogiwara, H., Tominaga, Y., Ito, K., Ando, O., Tsuta, K., Mizukami, T., Shimada, Y., Isomura, H., Komachi, M., Furuta, K., Watanabe, S., Nakano, T., Yokota, J., & Kohno, T. (2013). A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer research, 73(17), 5508–5518. https://doi.org/10.1158/0008-5472.CAN-12-4593
21 Hoffman, G. R., Rahal, R., Buxton, F., Xiang, K., McAllister, G., Frias, E., Bagdasarian, L., Huber, J., Lindeman, A., Chen, D., Romero, R., Ramadan, N., Phadke, T., Haas, K., Jaskelioff, M., Wilson, B. G., Meyer, M. J., Saenz-Vash, V., Zhai, H., Myer, V. E., … Jagani, Z. (2014). Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 3128–3133. https://doi.org/10.1073/pnas.1316793111
22. Papillon, J., Nakajima, K., Adair, C. D., Hempel, J., Jouk, A. O., Karki, R. G., Mathieu, S., Möbitz, H., Ntaganda, R., Smith, T., Visser, M., Hill, S. E., Hurtado, F. K., Chenail, G., Bhang, H. C., Bric, A., Xiang, K., Bushold, G., Gilbert, T., Vattay, A., … Jagani, Z. (2018). Discovery of Orally Active Inhibitors of Brahma Homolog (BRM)/SMARCA2 ATPase Activity for the Treatment of Brahma Related Gene 1 (BRG1)/SMARCA4-Mutant Cancers. Journal of medicinal chemistry, 61(22), 10155–10172. https://doi.org/10.1021/acs.jmedchem.8b01318
23. Martin, L. J., Koegl, M., Bader, G., Cockcroft, X.-L., Fedorov, O., Fiegen, D., … McConnell, D. (2016). Structure-Based Design of an in Vivo Active Selective BRD9 Inhibitor. Journal of Medicinal Chemistry, 59(10), 4462–4475.doi:10.1021/acs.jmedchem.5b0186
24. Bitler, B. G., Aird, K. M., Garipov, A., Li, H., Amatangelo, M., Kossenkov, A. V., Schultz, D. C., Liu, Q., Shih, I., Conejo-Garcia, J. R., Speicher, D. W., & Zhang, R. (2015). Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nature medicine, 21(3), 231–238. https://doi.org/10.1038/nm.3799
Have feedback? Tweet or DM me @blackseedbio on X, or contact me here.
If you liked this article and want to get notified when updates are available, consider subscribing (it's free, and you can unsubscribe at any time).
If you would like to donate to support the website, please click here.